Physician-focused algorithm in medicine
About us
We are a research group affiliated with the Institute of Clinical Chemistry at the Inselspital, University Hospital, and the University of Bern.
Closely linked to the Centre for Laboratory Medicine, our team is dedicated to bridge the translation gap between diagnostic innovation and clinical practice.
Vision
Our vision is to explore and improve what diagnostic tools look like, how they are developed and validated, how they are used in clinical care, and how their results are presented and communicated. We believe that the future of diagnostic medicine can be realized through a structured, interdisciplinary approach—combining clinical insight, laboratory expertise, translational knowledge, data science, and implementation research.
By collaborating with partners across clinical medicine, translational science, epidemiology, biostatistics, and AI, we aim to create diagnostic tools that are innovative, practical, equitable, and ready for real-world use.

Our Applications
TORADI-HIT
TORADI-HIT is a user-friendly machine learning algorithm that integrates clinical and laboratory data to diagnose heparin-induced thrombocytopenia (HIT). It outperforms current diagnostic methods and has been externally validated. The algorithm is designed to support fast and reliable decision-making—especially when time is critical.

MBD-Check
MBD-Check is an interpretable machine learning decision support tool for the preoperative screening of mild bleeding disorders. It is designed to simplify healthcare processes by reducing unnecessary referrals and improving patient care. The tool has demonstrated high diagnostic accuracy and was rated as user-friendly by clinicians.

H/PF4 Insight
In contrast to the standard positive/negative interpretation, this calculator translates anti-heparin/PF4 immunoassay results into clinically meaningful information.

ILVA
ILVA – the Insel Laboratory Verification App – is a web application for medical laboratories that automatically generates verification reports for certification and accreditation purposes, in accordance with the CLSI EP15-A3 and CLSI EP09-A3 guidelines.

SARS-CoV-2 Antibody Study Network
This web application visualizes the citation network of over 900 studies evaluating the diagnostic performance of SARS-CoV-2 antibody assays. It is part of a research project investigating how study design influences publication success and diagnostic accuracy measures.

How differently do similar antibody tests perform?
Curious how well your SARS-CoV-2 antibody test performs? This tool allows you to explore and pool data from over 900 studies—covering more than 300 different assays—to assess the diagnostic performance of specific SARS-CoV-2 antibody tests.
People
Michael Nagler, Prof. Dr. Dr. med.
Principal Investigator
Michael Nagler is a physician-scientist with extensive experience in clinical medicine, laboratory medicine, and epidemiology. His vision is to bridge the persistent translation gap between diagnostic innovation and clinical practice by building a platform that integrates the infrastructure, expertise, and technology needed for real-world implementation.

Henning Nilius, MD
Research associate
Henning Nilius is a physician and epidemiologist specializing in laboratory medicine, companion diagnostics, machine learning, and applied statistics. His research aims to unlock the full diagnostic potential of laboratory tests by applying advanced statistical and machine learning methods to maximize their clinical value.

Michael Horn, PhD
Diagnostic specialists
Michael Horn, PhD, is an experienced immunologist with a strong background in medical laboratory diagnostics. His passion lies in improving laboratory tests to better address a wide range of clinical questions—in close collaboration with experts from various clinical specialties.

Christof Schild, PhD
Diagnostic specialists
Christof Schild is a Biochemist with specialization in clinical chemistry FAMH. His passion is to develop and provide highly accurate, robust, and precise laboratory tests that directly support patient care.

Andrea Karolin, PhD
Diagnostic specialists
Missing description text...

Team
- Fammi Parokkaran, PhD student
- Robin Boss, MD
- Sarah Anna Kuonen, MD
Alumni
- Lukas Kuster, Dr. med.
- Samra Naas, Dr. med.
- Angelika Hammerer-Lercher, Dr. med.
- Annika Burger, Dr. med.
- Laura Boschetti, Dr. med.
- Tamara Mertins, Dr. med.
- Vepusha Sathanantham, Dr. med.
- Daria Eppenberger, Dr. med.
- Michael Esteves Pereira, Dr. med.
- Jonas Kaufmann, Dr. med.
- Luca Pellegrini, Dr. med.
- Tamana Meihandoest, MSc
- Guido Willekens, MSc
- Nathan Wolfensberger, Dr. med.
- Anna Wieland-Greguare, Dr. med.
- Andriyana Bankova, Dr. med.
- Raphael Müllner, MSc
Publications / follow us
Current projects
RADI-HIT Study
The RADI-HIT Study is an SNSF-funded study aiming to implement and validate a diagnostic algorithm in real-world settings. Its five core objectives are: (1) integration into hospital IT systems, (2) regulatory approval under the EU IVDR, (3) external validation in four European countries, (4) evaluation of usability and clinical utility in intensive care, and (5) assessment of impact on clinical outcomes and workflow. The study serves as a case study for successful real-world implementation of diagnostic algorithms.
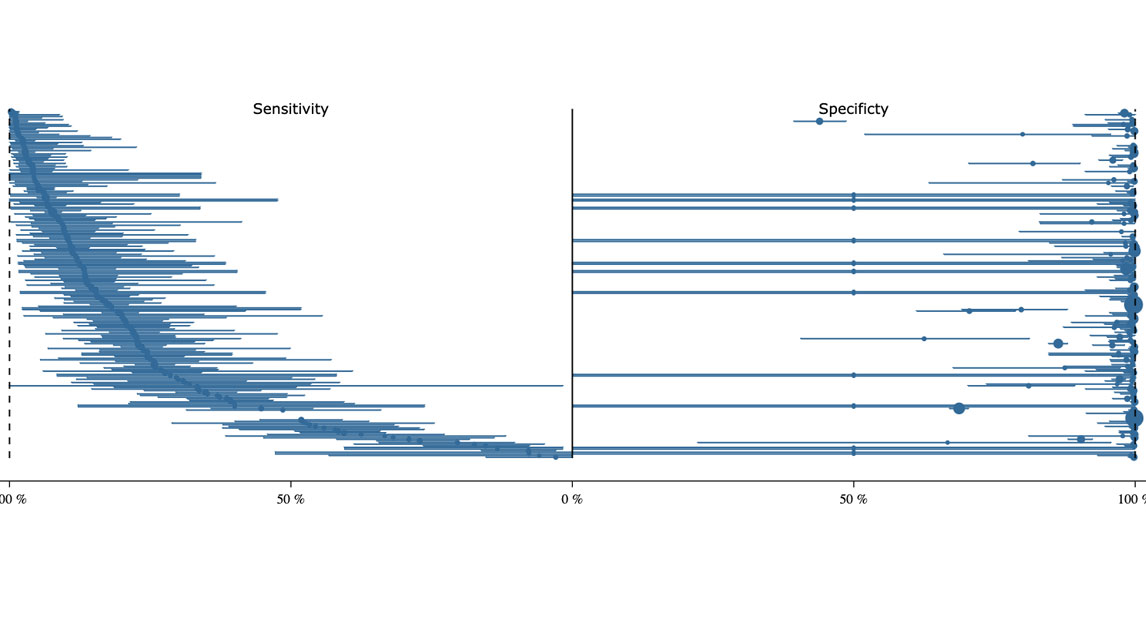
Bias in diagnostic research
While drug development follows a well-established translational pathway, diagnostic innovation still lacks such structured standards. This results in two major problems: First, there is a significant translation gap—very few diagnostic innovations from research reach clinical practice. Second, the real-world performance of established diagnostic tests is often far lower than assumed. Through a series of clinical studies, laboratory investigations, and meta-analyses, we systematically examine sources of bias and barriers to implementation. This work forms the basis for a methodological framework and practical toolbox to support the development, validation, and clinical integration of diagnostic innovations—particularly machine learning algorithms.
Definition of 'Healthy'
Diagnostic innovations—especially those envisioned in personalized medicine and concepts like the “digital twin”—must go beyond identifying clearly ill individuals. They must also detect subtle changes in people who appear healthy, revealing early signs of disease development or predisposition. Achieving this requires systematic study of apparently healthy individuals—an area still underexplored. To address this gap, we have established a laboratory cohort in an initial phase, distinguished by a large volume of precisely defined clinical and laboratory data.